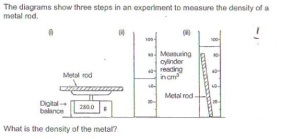
The diagram shows three steps in an experiment to measure the density of a metal rod.
One has to simply remember the formula of density in order to solve this question.
Density is defined as mass per unit volume
in this case, mass is 280g , and volume is 40cm^3
Hence, density= 280/40=7g/cm^3
Add on: It might be worthwhile to note that
Higher density objects will sink while low density object floats.
Find the density of sand, with only the information given below. Note that density of water is 1g/cm^3 and that the beakers are identical and filled to the brim . ( FIG 1.2)
Highlight black box for answer.
Lets start by listing out the variables in the question
- density of water
- volume of beaker/water
- mass of water
- mass of sand
- density of sand
- volume of sand
Of those listed, we do not know what is the
- volume of water
- mass of sand
- volume of sand
- density of sand
Those are the points we should tackle first
Looking at all the available information, we can easy find the volume of the water/beaker(1).
With ρ=m/v,
v=500×1 =500 cm^3.
Now, lets find the mass of sand,(2)
1200-308=892g
Now, to find the volume of sand, (3)
Lets first find the total mass of sand and water,
1500-308=1192g
Then, we find the mass of water in the last beaker,
1192-892=300g
volume of 300g of water= 300cm^3
This means that in the last beaker, the water itself takes up 300cm^3 of space, and sand takes up the rest of the volume of the beaker.
Now, to find the volume of the sand, we simply take ”Volume of beaker- Volume of water in beaker” =
500-300=200cm^3
Hence, density of sand = 892/200=4.4g/cm^3
I hope that was a clear enough explanation.